Professional Manufacturer of Biomagnetic Beads
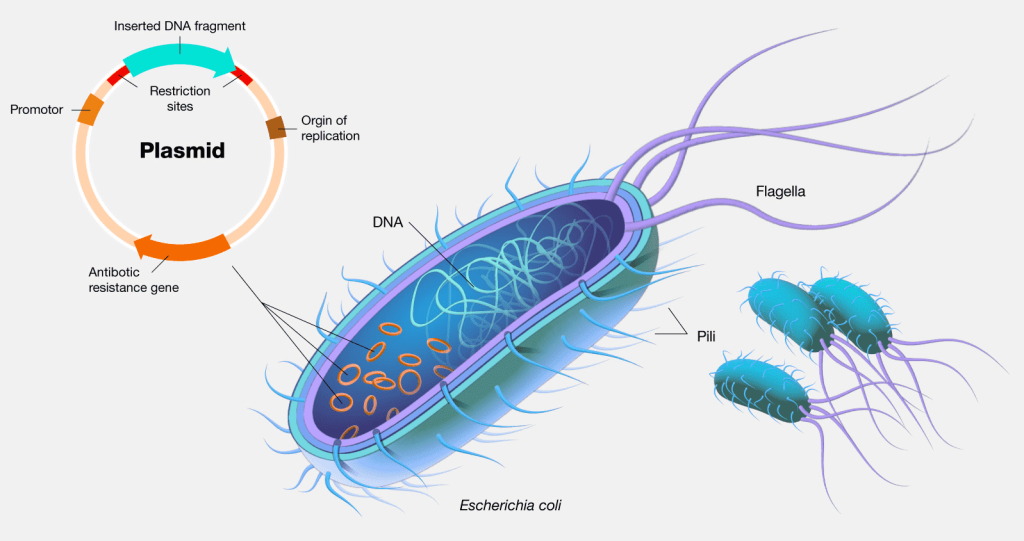
The use of endotoxin-free plasmids should be considered when the extracted plasmid DNA is used for transfection of primary, suspension, or sensitive cells, gene silencing studies, cell injection micromanipulation, gene therapy or DNA vaccines,and other extremely demanding experiments.
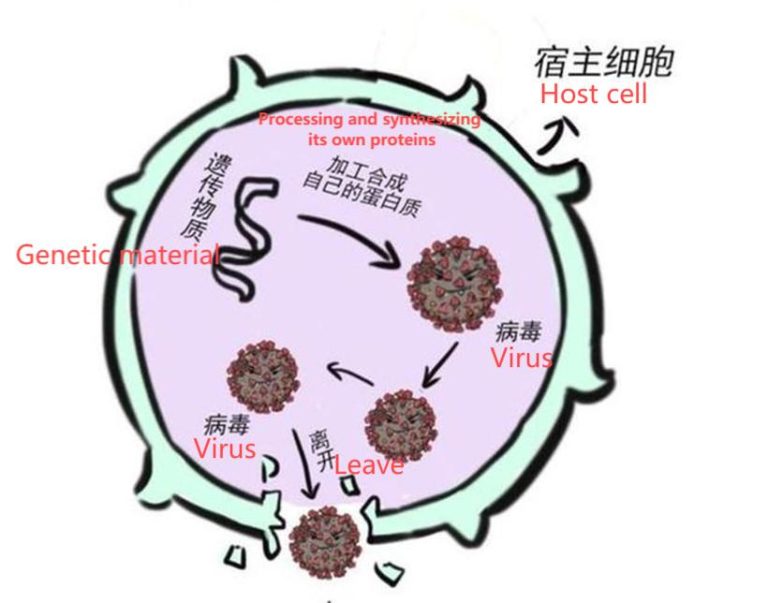
Plasmid de-endotoxinization refers to the step of removing endotoxin (lipopolysaccharide, LPS) during the purification of plasmid DNA. Endotoxin is a lipopolysaccharide and protein complex in the cell wall of Gram-negative bacteria that is released when the bacteria lyse. The presence of endotoxin greatly affects the transfection efficiency and may activate non-specific reactions of immune cells, resulting in false positives in the experiment. Therefore, in order to ensure a high transfection rate and high survival of transfected cells, endotoxin must be removed from the process of plasmid preparation.
The importance of endotoxin removal is:
1.Affect the transfection efficiency: endotoxin will reduce the transfection efficiency of primary cells and sensitive cultured cells, and interfere with the in vitro transfection of immune cells.
2.Activate immune response: endotoxin can also lead to fever, hypotension, respiratory distress, intravascular coagulation, and endotoxic shock syndrome, endotoxin can stimulate the synthesis of inflammatory factors IL-1, IL-6, IL-10, and TNF-α by activating TLR4.
3,.Drug regulatory requirements: The State Drug Administration stipulates that the content of endotoxin in DNA vaccine products should not be higher than 0.01 EU/μg, and the dose for personal use should not exceed 20 EU.
There are two strategies for endotoxin removal:
1. Removal at the binding stage: endotoxin is removed at the plasmid DNA binding stage.
2. Removal after elution: remove endotoxin after plasmid DNA elution.
The current endotoxin removal methods mainly include:
1. Non-selective removal: such as adsorption, ultrafiltration, and exclusion chromatography.
2. Selective precipitation: e.g., using polymyxin B, histidines, and poly-L-lysine affinity chromatography.
3. Hydrophobic interaction chromatography and ion exchange chromatography.
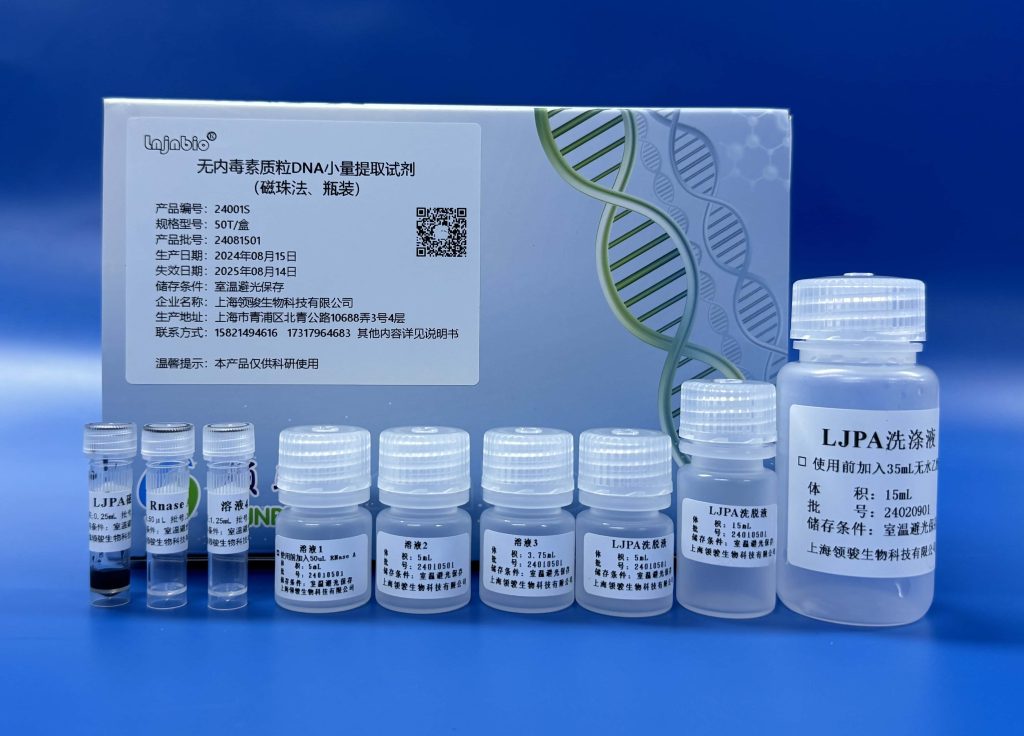
Our endotoxin-free plasmid DNA extraction kit (magnetic bead method) adopts a unique solution formula that requires only a few simple washes to remove endotoxin, proteins, polysaccharides, and other impurities, and then obtains high-quality plasmid DNA. This product extracts and purifies plasmid DNA with little damage to plasmids, high extraction concentration, and a high proportion of super-helix. The extracted plasmid DNA can be used for digestion, PCR, sequencing, ligation,n and transformation.
Supplier
Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid extraction and purification, protein purification, cell separation, chemiluminescence and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, and so on. We not only provide products but also can undertake OEM, ODM, and other needs.If you have a related need, please feel free to contact us at sales01@lingjunbio.com.
- The future of magnetic bead raw materials in the current trade barrier war between China and the United States
- 2025 IVD raw material market research report
- How long can whole blood samples be frozen?
- New era of biotechnology: AI and policy drive, innovative drug research and development to welcome the golden age
- Market research report of chemiluminescent magnetic beads(2025)