Professional Manufacturer of Biomagnetic Beads
The first step in extracting viral nucleic acids is to isolate viral particles from a sample infected with the virus, and then purify nucleic acids (DNA or RNA) from these viral particles. Viral nucleic acids are present inside viral particles, while viral particles can be present inside or outside the cells, which depends on the stage of the viral life cycle.
Extracellular viral particles
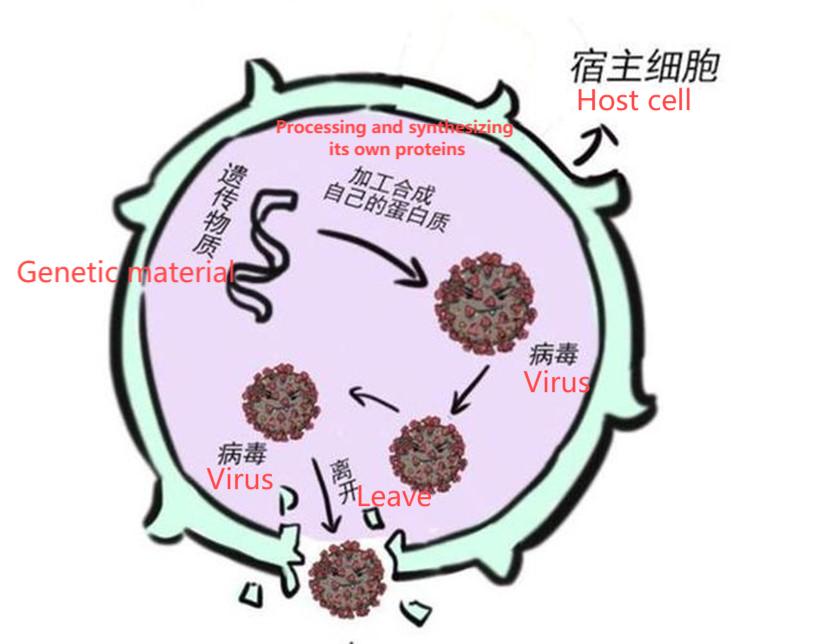
During the infection process, viruses can replicate and assemble into mature viral particles inside host cells. Once mature, these viral particles are released by cell lysis or budding and distributed in body fluids, secretions or the environment.
Extracellular viral particles contain intact viral nucleic acids, which can be released from viral particles by physical and chemical methods for subsequent purification and analysis.
Intracellular viral particles
In the early stages of the viral life cycle, viral nucleic acids may exist directly in the cytoplasm or nucleus, participating in the replication and transcription of the viral genome.
If the goal is to extract intracellular viral nucleic acids, the host cell membrane needs to be destroyed first to release the viral particles, and then the nucleic acids can be extracted from these viral particles.
Basic steps for viral nucleic acid extraction
Sample processing: lyse cells to release viral particles.
Precipitation or concentration of viral particles: use centrifugation or other methods to separate viral particles from the sample.
Lysis of viral particles: use chemical reagents to destroy the protein capsid of the virus and release nucleic acids.
Nucleic acid purification: remove proteins, lipids and other impurities to purify high-purity viral nucleic acids.
Nucleic acid quantification and quality assessment: use ultraviolet spectroscopy or fluorescence quantification and other techniques to quantify the extracted nucleic acids.
In actual laboratory operations, whether it is intracellular or extracellular viral nucleic acid extraction, similar lysis, purification and protection measures are used to ensure the quality and integrity of the nucleic acids. However, the specific operational details and pretreatment steps need to be adjusted according to the different sources of nucleic acids.
Supplier Introduction

Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid isolation and purification, protein purification, cell separation, chemiluminescence and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, etc. We not only provide products but also can undertake OEM, ODM, and other needs. If you have related needs, please feel free to contact us at sales01@lingjunbio.com.